Multiple Choice
What is the hydronium ion concentration in an aqueous solution prepared by mixing 50.00 mL of 0.10 mol L-1 HCN with 50.00 mL of 0.010 mol L-1 NaCN? Assume that the volumes of the solutions are additive and that Ka = 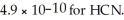
A) 4.9 × 10-11 mol L-1
B) 4.9 × 10-10 mol L-1
C) 4.9 × 10-9 mol L-1
D) 7.0 × 10-6 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q74: What will the pH at the neutralization
Q75: A buffer was prepared by adding 2.4
Q76: An aqueous solution containing equimolar amounts of
Q77: 25 mL of 0.10 M aqueous acetic
Q78: If 30.0 mmol HCl(g)is added to 1.00
Q80: For HClO<sub>2</sub>,K<sub>a</sub> = 1.2 × 10<sup>-2</sup>.What is
Q81: What is the pH of a 1.0
Q82: An aqueous solution of an unknown acid
Q83: What is the pH of the resulting
Q84: A handbook states that to prepare a