Multiple Choice
How many millilitres of 0.0850 mol L-1 NaOH(aq) are required to titrate 25.0 mL of 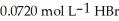 (aq) to the equivalence point?
(aq) to the equivalence point?
A) 21.2
B) 0.245
C) 3.92
D) 0.153
E) 29.5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q96: Twenty-five milliliters of 0.10 M HCl(aq)is titrated
Q97: What volume in mL of 2.0 M
Q98: The neutralization of a weak acid with
Q99: What volume in mL of 0.05 M
Q100: The solution that is added from the
Q102: The titration curve for 10.0 mL of
Q103: Phenolphthalein may be used as an indicator
Q104: Calculate the pH of a 1.00 L
Q105: The common ion in an aqueous solution
Q106: What is the pH of a solution