Multiple Choice
How many millilitres of 0.120 mol L-1 NaOH(aq) are required to titrate 50.0 mL of 0.0998 mol L-1 aqueous butanoic acid to the equivalence point? Butanoic acid is monoprotic.The 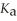
Of butanoic acid is 1.5 × 10-5.
A) 4.90
B) 50.0
C) 41.6
D) 60.1
E) 4.65
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q82: An aqueous solution of an unknown acid
Q83: What is the pH of the resulting
Q84: A handbook states that to prepare a
Q85: The common ion in an aqueous solution
Q86: Determine the [C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>-</sup>] of the following aqueous
Q88: A weak acid has K<sub>a</sub> = 4.2
Q89: The following compounds are available as 0.10
Q90: What is the pH of an aqueous
Q91: What is the [H<sub>3</sub>O<sup>+</sup>] of an aqueous
Q92: How many mL of 0.200 M aqueous