Multiple Choice
A 25.0 mL sample of 0.150 mol L-1 aqueous butanoic acid is titrated with a 0.150 mol L-1 NaOH(aq) solution.What is the pH before any base is added? Butanoic acid is monoprotic.The 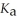 Of butanoic acid Is 1.5 × 10-5.
Of butanoic acid Is 1.5 × 10-5.
A) 2.82
B) 1.5 × 10-3
C) 4.82
D) 4.00
E) 1.0 × 104
Correct Answer:

Verified
Correct Answer:
Verified
Q22: What is the pH of an aqueous
Q23: A buffer is 0.282 M C<sub>6</sub>H<sub>5</sub>COOH(aq)and 0.282
Q24: Ten milliliters of 0.10 M NH<sub>3</sub>(aq)(K<sub>b</sub> =
Q25: What is the pH at the equivalence
Q26: What is the pH of an aqueous
Q28: A weak acid has K<sub>a</sub> = 1.00
Q29: Sodium hypochlorite,NaOCl,is the active ingredient in household
Q30: Phenol red indicator changes from yellow to
Q31: Which of the following mixtures would you
Q32: What is the pH of an aqueous