Multiple Choice
A concentrated buffer of pH 8.0 is added to an equal volume of an aqueous solution that is 0.080 M in each of the ions Zn2+,Ni2+,and Mn2+.The expected precipitate would consist of ________.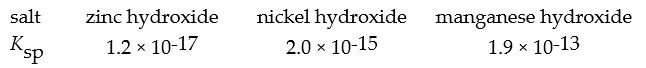
A) Zn(OH) 2,Ni(OH) 2 and Mn(OH) 2
B) Ni(OH) 2 and Mn(OH) 2
C) Mn(OH) 2
D) Ni(OH) 2 and Zn(OH) 2
E) Zn(OH) 2
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Fe(OH)<sub>3</sub> is most soluble in which aqueous
Q24: When equal volumes of the indicated aqueous
Q25: When 200 mL of 0.10 M BaCl<sub>2</sub>(aq)is
Q26: The K<sub>sp</sub> of AgCl is 1.7 ×
Q27: Calculate the solubility (in g L<sup>-1</sup>)of calcium
Q29: Write the solubility product constant for KAl(SO<sub>4</sub>)<sub>2</sub>(s)?<br>A)([K<sup>+</sup>]
Q30: Which of the following soil additives would
Q31: What is the minimum concentration of CN<sup>-</sup>(aq)that
Q32: What is the approximate concentration of free
Q33: Which of the following has the largest