Multiple Choice
What is the molar solubility of Mg(OH) 2(s) in a basic aqueous solution with a pH of 12.50? Ksp for Mg(OH) 2(s) is 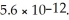
A) 1.8 × 10-10 mol L-1
B) 5.6 × 10-9 mol L-1
C) 2.4 × 10-6 mol L-1
D) 1.1 × 10-4 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: What is the approximate concentration of free
Q33: Which of the following has the largest
Q34: The solubility of CaF<sub>2</sub> is 0.00021 mole
Q35: Predict the molar solubility of the following
Q36: The solubility product constant of M<sub>2</sub>CrO<sub>4</sub>(s)is 7.11
Q38: Write the solubility product constant expression for
Q39: What is the molar solubility of barium
Q40: The molar solubility of SrSO<sub>4</sub> (K<sub>sp</sub> =
Q41: If iron(III)acetate is added to be 1
Q42: When solid silver chloride is shaken with