Multiple Choice
Calculate the molar solubility of thallium chloride (TlCl) in 0.30 mol L-1 NaCl(aq) at 25 °C.Ksp for TlCl(s) is 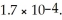
A) 5.1 × 10-5 mol L-1
B) 5.7 × 10-4 mol L-1
C) 7.1 × 10-3 mol L-1
D) 1.3 × 10-2 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q1: To a saturated aqueous solution of barium
Q3: Which of the following is least soluble?<br>A)NiS
Q4: To a concentrated buffer of pH 9.0
Q5: What is the concentration of free Zn<sup>2+</sup>(aq)if
Q6: The following table lists five compounds and
Q7: Which of the following should dissolve the
Q8: In a qualitative cation analysis,the unknown ion
Q9: What is the free Cu<sup>2+</sup>(aq)concentration if 0.020
Q10: The solubility of cerium iodate,Ce(IO<sub>3</sub>)<sub>3</sub>,molar mass =
Q11: An aqueous solution contains [Ba<sup>2+</sup>] = 5.0