Multiple Choice
A voltaic cell is constructed with two 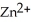 -Zn electrodes,where the half-reaction is
-Zn electrodes,where the half-reaction is 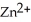 (aq) +
(aq) + 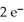 → Zn(s) E° = -0.763 V
→ Zn(s) E° = -0.763 V
The concentrations of zinc ion in the two compartments are 5.50 mol L-1 and 1.11 × 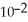
Mol L-1,respectively.The cell emf is ________ V.
A) -1.54 × 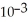
B) -378
C) 0.0798
D) 0.160
E) -0.761
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Consider the following standard reduction potentials:<br>Ni<sup>2+</sup>(aq)+ 2
Q76: A voltaic cell is constructed with two
Q77: Choose the INCORRECT statement.<br>A)Corrosion of metals is
Q78: For the galvanic cell reaction,expressed below using
Q79: What is the shorthand notation that represents
Q81: The E<sub>cell</sub> for the following concentration cell
Q82: The net reaction in a voltaic cell
Q83: Consider the following reaction:<br>2 Al(s)+ 3 Ni<sup>2+</sup>(aq)→
Q84: In the electrolysis of water,oxygen is evolved
Q85: Choose the INCORRECT statement.<br>A)A double vertical line