Multiple Choice
Data for the reaction A + B → C are given below.Find the rate constant for this system. 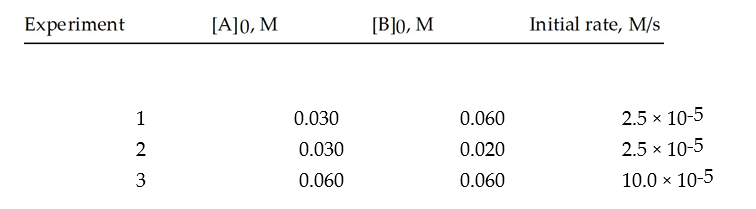
A) 2.8 × 10-2 M-1s-1
B) 2.8 × 10-2 Ms-1
C) 2.8 × 10-2 M2s-1
D) 1.7 × 10-3 M-1s-1
E) 1.7 × 10-3 Ms-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the order of reaction for
Q2: Energy of activation has no effect on
Q3: The decomposition of dinitrogen pentoxide is described
Q5: A reaction is first order.If its initial
Q6: Activation energy is:<br>I.the minimum kinetic energy required
Q7: According to the collision theory in gaseous
Q8: The rate of disappearance of HBr in
Q9: What is the overall reaction order for
Q10: Define activation energy.<br>A)the difference between the energy
Q11: For a reaction Rate = k[A][B]<sup>2</sup>,what factor