Multiple Choice
The decomposition of 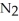
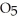 In solution in carbon tetrachloride proceeds via the reaction
In solution in carbon tetrachloride proceeds via the reaction 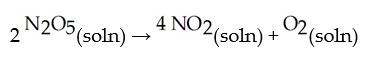
The reaction is first order and has a rate constant of 4.82 × 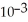
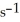
At 64 °C.If the reaction is initiated with 0.058 mol in a 1.00 L vessel,how many moles remain after 151 s?
A) 0.055 mol L-1
B) 0.060 mol L-1
C) 0.028 mol L-1
D) 12 mol L-1
E) 2.0 × 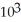 mol L-1
mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Which of the following situations involves a
Q26: In the reaction C<sub>4</sub>H<sub>9</sub>Cl(aq)+ H<sub>2</sub>O(l)→ C<sub>4</sub>H<sub>9</sub>OH(aq)+ HCl(aq)the
Q27: Carbon-14,which is present in all living tissue,radioactively
Q28: For the reaction: C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub> + 3 KI
Q29: For a reaction with a second order
Q31: A catalyst alters the rate of a
Q32: The following reaction is first order,C<sub>2</sub>H<sub>6</sub> →
Q33: Adding a catalyst lowers the activation energy
Q34: Activation energy is:<br>A)energy at the bottom of
Q35: In the first-order,reaction A → products,[A] =