Multiple Choice
The production of lead is a two step process:
2 PbS(s) + 3 O2(g) 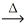 2 PbO(s) + 2 SO2(g)
2 PbO(s) + 2 SO2(g)
2 PbO(s) + C(s) 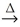 2 Pb(l) + CO2(g)
2 Pb(l) + CO2(g)
How much ore that is 5.2% PbS is necessary to produce 12.0 kg of Pb?
A) 0.721 kg
B) 200 kg
C) 2.67 kg
D) 133 kg
E) 267 kg
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Finish the following reaction:<br>SnO<sub>2</sub> + NaOH(aq)+ H<sub>2</sub>O(l)→<br>A)SnO<sub>2</sub>(s)+
Q42: Which of the following statements about the
Q43: Predict the shape of cyanogen, (CN)<sub>2</sub>.<br>A)linear<br>B)bent<br>C)tetrahedral<br>D)trigonal pyramidal<br>E)square
Q44: In diborane,B<sub>2</sub>H<sub>6</sub>,the hybridization of each boron is:<br>A)sp<sup>3</sup><br>B)sp<sup>2</sup><br>C)sp<sup>3</sup><sup>d</sup><br>D)sp<br>E)sp<sup>3</sup><sup>d</sup><sup>2</sup>
Q45: What is the pH of a solution
Q47: Bronze is 90% Cu and 10% Sn
Q48: Which of the following is a weak
Q49: The atomic radius of calcium is smaller
Q50: An aqueous solution of boric acid can
Q51: How much tin is needed to make