Multiple Choice
Write a balanced half equation for the following couple: 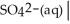 S(s) (acidic)
S(s) (acidic)
A) SO42-(aq) + 2 H+(aq) + 2 e- → S(s) + H2O(l)
B) SO42-(aq) + H2O(l) + 4e- → S(s) + 2 H+(aq)
C) SO42-(aq) + 2 H+(aq) + 2 e- → S(s) + H2SO4(aq)
D) SO42-(aq) + 8 H+(aq) + 6 e- → S(s) + 4 H2O(l)
E) SO42-(aq) + 8 H+(aq) → S(s) + 4 H2O(l) + 6 e-
Correct Answer:

Verified
Correct Answer:
Verified
Q10: The VSEPR theory predicts molecular shapes for
Q11: Give the molecular shape of NO<sub>3</sub><sup>-</sup>.<br>A)trigonal pyramidal<br>B)trigonal
Q12: The compound of which P<sub>4</sub>O<sub>6</sub> is the
Q13: The electron group geometry for XeF<sub>2</sub> is
Q14: Choose the INCORRECT name/formula combination.<br>A)hydrogen chloride HClO<br>B)sodium
Q16: Pyrosulfuric is also called disulfuric H<sub>2</sub>S<sub>2</sub>O<sub>7</sub>.Pyrosulfuric acid
Q17: One of the natural processes that destroys
Q18: What is the molecular shape for XeOF<sub>2</sub>?<br>A)trigonal
Q19: Give the molecular shape of SO<sub>4</sub><sup>2-</sup>.<br>A)trigonal pyramidal<br>B)trigonal
Q20: "Halogen" means "soluble salts."