Multiple Choice
Write a balanced half equation for the following couple: 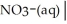 NH4+(aq) (acidic)
NH4+(aq) (acidic)
A) NO3-(aq) + 3 H2O(l) → NH4+(aq) + 10 H+(aq) + 8 e-
B) NO3-(aq) + 4 H+(aq) + 3 e- → NH4+(aq) + 3 H2O(l)
C) NO3-(aq) + 4 H+(aq) → NH4+(aq) + 3 H2O(l) + e-
D) NO3-(aq) + 8 H+(aq) + 8 e- → NH4+(aq) + 3 H2O(l)
E) NO3-(aq) + 10 H+(aq) + 8 e- → NH4+(aq) + 3 H2O(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q35: What is one of the important uses
Q36: The pollution process in which oxygen depletion
Q37: Which element is in a family that
Q38: In a reaction between XeO<sub>3</sub>(aq)and Mn<sup>2+</sup>(aq)that occurred
Q39: What is the correct completion of the
Q41: What is the skeletal structure for chloric
Q42: Choose the INCORRECT statement.<br>A)Chlorofluorocarbon production has been
Q43: What volume of 17 M nitric acid
Q44: HI(g)has a ΔG<sub>f</sub>° that is small and
Q45: Halogen molecules are diatomic.