Multiple Choice
Write a balanced half equation for the following couple: 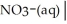 NO2-(aq) (basic)
NO2-(aq) (basic)
A) NO3-(aq) + 2 H+(aq) + 2 e- → NO2-(aq) + H2O(l)
B) NO3-(aq) + 2 H2O(l) + 2 e- → NO2-(aq) + 2 OH-(aq) + 2 H2O(l)
C) NO3-(aq) + H2O(l) + 2 e- → NO2-(aq) + 2 OH-(aq)
D) NO3-(aq) + H2O(l) + e- → NO2-(aq) + OH-(aq)
E) NO3-v + H2(g) + e- → NO2-(aq) + OH-(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q72: "Chlorine-type" laundry bleaches are in reality aqueous
Q73: Which of the following exists in more
Q74: Choose the INCORRECT statement.<br>A)Xenon compounds in several
Q75: In certain automotive catalytic converters,one step involves
Q76: A bleaching solution can be prepared by
Q78: Bromine can be produced by:<br>A)oxidizing Cl<sub>2</sub> with
Q79: Which of the following forms a complete
Q80: Choose the INCORRECT statement.<br>A)H<sub>2</sub>O has a high
Q81: Choose the INCORRECT statement.<br>A)The Frasch process is
Q82: "Red" phosphorus is insoluble in carbon disulfide,has