Multiple Choice
On forming the nuclide from the protons,neutrons and electrons,the mass loss per nucleon for the 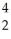 He is 0.00759 amu,whereas that for
He is 0.00759 amu,whereas that for 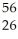 Fe is 0.00944 amu.This means that:
Fe is 0.00944 amu.This means that:
A) 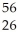 Fe undergoes nuclear fission
Fe undergoes nuclear fission
B) 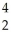 He is more stable than
He is more stable than 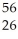 Fe
Fe
C) thirteen 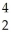 He nuclei could react to form one
He nuclei could react to form one 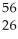 Fe nucleus
Fe nucleus
D) 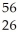 Fe is more stable than
Fe is more stable than 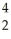 He
He
E) 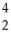 He weighs more than
He weighs more than 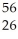 Fe
Fe
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Radioactivity is a first order kinetics process.
Q9: Which of the following nuclides is most
Q10: A certain radioactive series starts with <img
Q11: A wooden artifact is subjected to radiocarbon
Q12: The observation that a 30 g sample
Q15: A certain isotope has a half-life of
Q16: When <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="When Sr
Q17: Most periodic tables indicate the elements for
Q18: A freshly prepared sample of curium-243 undergoes
Q47: Carbon-11 is used in medical imaging. The