Multiple Choice
In the reaction 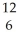 C +
C + 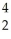 He →
He → 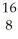 O
O
how much energy is produced per gram of carbon used?
Masses,in unified atomic mass units are: 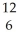 C= 12.00000,
C= 12.00000, 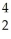 He= 4.00260,
He= 4.00260, 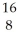 O = 15.99491.
O = 15.99491.
Express your answer in Joules/gram of carbon.
A) 2.10 × 1019 J/g
B) 5.76 × 1010 J/g
C) 7.77 × 104 J/g
D) 2.80 × 1011 J/g
E) 3.36 × 1015 J/g
Correct Answer:

Verified
Correct Answer:
Verified
Q103: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt=" B decays via
Q104: Emission of which one of the following
Q105: The binding energy per nucleon is largest
Q106: Which of the following nuclear species would
Q107: A nuclide has a decay constant of
Q109: Potassium-40 decays to argon-40 with a half-life
Q110: Choose the possible products for the nuclear
Q111: Which of the following is true concerning
Q112: An isotope which has too high a
Q113: Choose the INCORRECT statement.<br>A)Fusion is the basis