True/False
An element has the following electron arrangement.
shell 1:2 electrons shell 2: 8 electrons shell 3:18 electrons shell 4:18 electrons shell 5:7 electrons
The Lewis structure for this element would be: 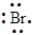
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: Sodium chlorate, an ingredient in many common
Q57: How many moles of oxygen would be
Q58: An isotope of gallium consisting of 31
Q59: What is the meaning of the subscripts
Q60: Based on the text periodic table and
Q62: When a particular solid sample is examined
Q63: Based on the text periodic table and
Q64: An elements with an electron arrangement of:<br>shell
Q65: Sodium is a highly reactive metal and
Q66: The image shown below represents an atomic