Multiple Choice
The heat of vaporization of water is 540 cal/g. What mass of water at 100.0 °C can be vaporized by the addition of 55.0 kcal of heat?
A) 0.19 g
B) 55 g
C) 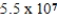 g
g
D) 102 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: The two compounds below have the same
Q41: Which of the following molecules cannot engage
Q42: The specific heat of ice is 0.480
Q43: In order to convert a pressure in
Q44: Helium (He)is used to fill balloons and
Q46: Potassium phosphate, K<sub>3</sub>PO<sub>4</sub>, is a solid at
Q47: Consider the following three substances. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg"
Q48: Below is the structure of the amino
Q49: Glycerol changes its shape to conform to
Q50: Convert 0.662 atm to millibars.<br>A)377 millibar<br>B)671 millibar<br>C)