Multiple Choice
When natural gas (predominantly methane, CH4) burns in air. The following reaction occurs. How much energy is involved in the combustion of 13.0 g of methane? 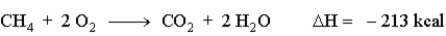
A) 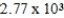 kcal
kcal
B) 16.4 kcal
C) 173 kcal
D) 0.979 kcal
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The process that converts the energy stored
Q2: How many moles of N<sub>2</sub> are required
Q3: An aqueous solution of sodium nitrate (NaNO<sub>3</sub>)
Q5: Consider the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt="Consider
Q6: Consider the following energy diagram showing a
Q7: Complete each of the following sentences using
Q8: Copper reacts with nitric acid as given
Q9: Reactions that have a low energy of
Q10: The following reaction could be classified as
Q11: The following represents a typical energy diagram.