Multiple Choice
If the reaction given below occurs and pure A and B were mixed, which of the following would take place as equilibrium was established?
A + B 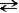 C
C
A) The concentration of C would increase for a time, then remain constant.
B) The concentration of A would increase for a time, then decrease.
C) The concentration of B would increase for a time, then remain constant.
D) Both a and c will occur.
Correct Answer:

Verified
Correct Answer:
Verified
Q11: The Haber Process is a method for
Q39: Which of the following reactions is correctly
Q51: The following would be classified as a
Q52: Consider the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt="Consider
Q53: Photosynthesis and respiration are linked to each
Q54: Draw the energy diagram for the following
Q57: The combustion of glucose per gram produces
Q58: Which of the following the appropriate ending
Q59: Consider the following reaction. <br>NaOH(aq) + HCl(aq)
Q60: Which of the following is a physical