Multiple Choice
Consider a sample of water in a closed container. When the reaction H2O(l) 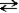 H2O(g)
H2O(g)
Has reached equilibrium, what can we say about any specific water molecule?
A) If it was present in the liquid it will always remain in the liquid.
B) If it was present in the gas it will always remain in the gas.
C) It will sometimes be in the liquid phase and sometimes in the gas phase.
D) Both a and b are true.
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Per gram _ produces the largest amount
Q15: Based on the following energy diagram, the
Q16: Complete each of the following sentences using
Q17: During the carbon cycle plants produce the
Q18: Consider the following image representing an equilibrium
Q20: One Hawaiian sweet roll contains:<br>2.5 g fat<br>18
Q21: When you inhale (breathe in), oxygen is
Q22: Decreasing the temperature will decrease the number
Q23: Complete each of the following sentences using
Q24: When magnesium reacts with nitrogen, the reaction