Short Answer
Copper reacts with nitric acid as given in the following reaction. 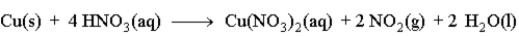 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
increase
decrease
no effect
-The copper metal is ground into a very fine powder. ______________________
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Consider the following image representing an equilibrium
Q29: Consider the following image. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt="Consider
Q30: Complete each of the following sentences using
Q31: Consider the following reaction. <br>NaOH(aq) + H<sub>2</sub>SO<sub>4</sub>(aq)
Q32: Which of the following is the best
Q34: Ethanol is produced industrially by the acid
Q35: Which of the following most closely defines
Q36: Copper reacts with nitric acid as given
Q37: In the equation for an exothermic reaction,
Q38: If the 'Nutrition Facts' on a food