Multiple Choice
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants:
Products: 
If the composition of the equilibrium mixture is as represented below 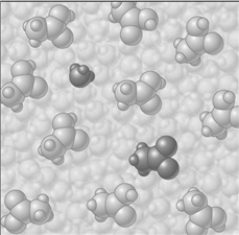
-CH3COOH is classified as
A) strong electrolyte
B) weak electrolyte
C) nonelectrolyte
Correct Answer:

Verified
Correct Answer:
Verified
Q13: The following represents the structure of a
Q14: Ascorbic acid (Vitamin C), H<sub>2</sub>C<sub>6</sub>H<sub>6</sub>O<sub>6</sub>, has a
Q15: A sodium hydroxide solution has a pH
Q17: Write the equation for the ionization of
Q19: Consider the following three buffer reactions.<br>Buffer 1:
Q20: Which of the following substances is amphiprotic?<br>A)HCO<sub>3</sub><sup>-</sup><br>B)HPO<sub>4</sub><sup>3-</sup><br>C)H<sub>2</sub>PO<sub>4</sub><sup>-</sup><br>D)All
Q21: Joshua has had high blood pressure for
Q22: Consider the following three buffer reactions.<br>Buffer 1:
Q23: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt=" Using the above
Q37: Which of the following is a cause