Multiple Choice
Consider the compounds given in the choices. Which would have the lowest melting point?
A) 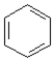
B) 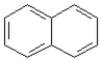
C) 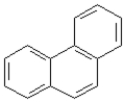
D) All have about the same melting point.
Correct Answer:

Verified
Correct Answer:
Verified
Q44: Consider the following structure. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt="Consider
Q45: In propyne, the arrangement of bonds around
Q46: Consider the following molecules. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt="Consider
Q47: What is line bond formula for the
Q48: What is the IUPAC name for the
Q50: The following compound would be classified as
Q51: Draw the condensed structural formula of 3-ethyl-3-methylhexane.
Q52: Examine the following structures. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt="Examine
Q53: The two compounds shown below have the
Q54: Consider the following molecule of the birth