Essay
Consider the following reaction: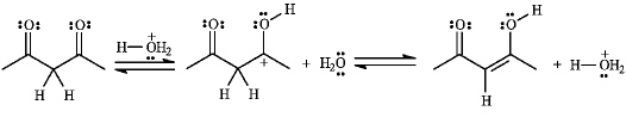
a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
b) If the second step of the reaction were the slow step, briefly explain how the values of 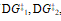 , and DG° would change.
, and DG° would change.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Instructions: Classify each structure below as a
Q6: Instructions: Add curved arrows to the following
Q7: How many monochlorosubstitution products are possible for
Q8: Instructions: Match each definition to one of
Q10: The structures below show the stepwise bond
Q14: In an organic reaction, which of the
Q16: Use the first step of the reaction
Q23: Use the first step of the reaction
Q24: Use the second and third steps of
Q38: Identify the functional groups present in each