Multiple Choice
Which is the strongest base (pKa values given for conjugate acid) ?
A) NH3 (pKa = 9.2)
B) CH3O- (pKa = 16)
C) 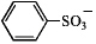
(pKa = -6.5)
D) CH3CO2- (pKa = 4.7)
E) H- (pKa = 35)
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Complete this acid-base reaction,and label the acid,base,conjugate
Q6: Circle the Lewis bases in the group
Q8: Among the following compounds which can function
Q9: In aromatic nitration reactions,nitric acid (HNO<sub>3</sub>)is used
Q10: What is the formal charge on the
Q11: Of the bonds found in <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4938/.jpg"
Q12: Explain why phenol has a lower pK<sub>a</sub>
Q17: Which of the following molecules would exhibit
Q37: Instructions: Indole is pleasant smelling in highly
Q39: Instructions: Consider the species below to answer