Multiple Choice
Calculate the average atomic mass of lithium using the following data: 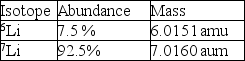
A) 6.51 amu
B) 6.02 amu
C) 6.94 amu
D) 7.02 amu
E) 6.50 amu
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: An average atom of uranium (U) is
Q53: Calculate the molar mass of Ba(NO<sub>3</sub>)<sub>2</sub>.<br>A) 199.3
Q77: A compound was discovered whose composition by
Q104: A sample of iron metal is
Q134: Calculate the percent composition by mass of
Q136: Acetylene gas, HCCH(g), can be generated
Q137: Calculate the mass of FeS formed
Q138: A 0.600 g sample of a compound
Q140: What is the coefficient of H<sub>2</sub>O
Q144: Refer to the (unbalanced)equation CS<sub>2</sub> +