Multiple Choice
Calculate the average atomic mass of silver using the following data: 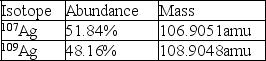
A) 106.91 amu
B) 108.00 amu
C) 107.90 amu
D) 107.87 amu
E) 108.90 amu
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Calculate the mass of C in 5.46
Q58: What is the coefficient of H<sub>2</sub>O
Q118: A method for producing pure copper metal
Q119: What mass of sodium nitrate would
Q120: Chlorine gas can be made from
Q120: Calculate the mass of O in 4.36
Q122: A compound with a percent composition by
Q125: Balance the following chemical equation:<br>Al(s)+ Co(NO<sub>3</sub>)<sub>2</sub>(aq)
Q128: If 0.274 moles of a substance weighs
Q171: What is the theoretical yield of