Essay
Nickel has a lower atomic mass than cobalt, even though it has a higher atomic number.One possible explanation is that one of the average atomic masses was miscalculated.In the case of cobalt, there is only one isotope: 100% 59Co at a mass of 58.9332 amu.For nickel, however, there are five isotopes as given in the table. 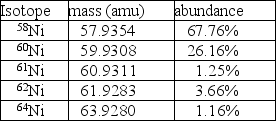 A.Using the data in the table, calculate the average atomic mass for nickel.
A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
Correct Answer:

Verified
A.58.70 amu
B.yes
C.Cobalt has 27 proton...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
B.yes
C.Cobalt has 27 proton...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q14: Which one of the following is
Q46: The reaction of 44.1 g of
Q52: A mass spectrometer works by ionizing atoms
Q54: How many fluorine atoms are there in
Q55: If 0.66 mole of a substance has
Q57: The molecular formula of aspirin is C<sub>9</sub>H<sub>8</sub>O<sub>4</sub>.How
Q60: A compound with a percent composition by
Q61: How many Mg atoms are present in
Q102: Calculate the mass of excess reagent
Q194: Which of the following NH<sub>3</sub> samples contains