Short Answer
A chemistry student determined the empirical formula for tungsten oxide (WxOy).To do so, he heated tungsten with oxygen in a crucible.The data that he recorded are shown below: 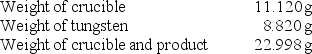 What is the empirical formula of tungsten oxide?
What is the empirical formula of tungsten oxide?
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A silver wire has a diameter of
Q6: Lithium metal reacts with nitrogen gas
Q73: Hydrogen chloride gas can be prepared
Q82: How many O atoms are in 4.39
Q93: What is the coefficient for O<sub>2</sub>
Q144: Refer to the (unbalanced)equation CS<sub>2</sub> +
Q146: Calculate the mass of 3.00 moles of
Q150: Formaldehyde has the formula CH<sub>2</sub>O.How many molecules
Q153: What is the mass of 0.0250 mol
Q178: What is the theoretical yield of