Multiple Choice
Identify the correct net ionic equation for the reaction that occurs when solutions of Pb(NO3) 2 and NH4Cl are mixed.
A) Pb(NO3) 2(aq) + 2NH4Cl(aq) NH4NO3(aq) + PbCl2(s)
B) Pb2+(aq) + 2Cl-(aq) PbCl2(s)
C) Pb2+(aq) + 2NO3- (aq) + 2NH 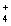 (aq) + 2Cl-(aq) 2NH
(aq) + 2Cl-(aq) 2NH 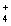 (aq) + 2NO3- (aq) + PbCl2(s)
(aq) + 2NO3- (aq) + PbCl2(s)
D) NH4+(aq) + NO3- (aq) 2NH4NO3(s)
E) No reaction occurs when the solutions are mixed.
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Which of the following will occur when
Q52: Which of the following compounds is a
Q75: Identify the major ionic species present in
Q91: The oxidation number of N in N<sub>2</sub>H<sub>4</sub>
Q98: Identify the correct net ionic equation
Q143: The common constituent in all acid solutions
Q163: A piece of lead metal was added
Q169: Identify the following compound as a strong
Q171: Write the balanced molecular and net ionic
Q172: The solubility of Ba(NO<sub>3</sub>)<sub>2</sub> is 130.5 grams