Multiple Choice
Calculate the standard enthalpy of formation of liquid methanol, CH3OH(l) , using the following information: 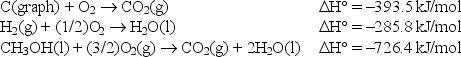
A) -1,691.5 kJ/mol
B) -238.7 kJ/mol
C) -47.1 kJ/mol
D) 47.1 kJ/mol
E) 1691.5 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q73: The heat capacity of 20.0 g of
Q74: When 0.560 g of Na(s)reacts with
Q75: The reaction that represents the standard
Q75: A feverish student weighing 75 kilograms was
Q76: Radiant energy is<br>A) the energy stored within
Q78: If 2Mg(s)+ O<sub>2</sub>(g) <span class="ql-formula" data-value="\rarr"><span
Q79: An endothermic reaction causes the surroundings to<br>A)warm
Q80: Find <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q82: Calculate the heat released (kJ)in the
Q119: Heat is<br>A) a measure of temperature.<br>B) a