Multiple Choice
A photon is roughly 1800 times more massive than an electron.If a proton and an electron have the same kinetic energy,
A) the wavelength of the photon will be about 1800 times longer than the wavelength of the electron.
B) the wavelength of the photon will be about 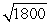 times longer than the wavelength of the electron.
times longer than the wavelength of the electron.
C) the wavelength of the photon will be roughly equal to the wavelength of the electron.
D) the wavelength of the electron will be about 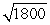 times longer than the wavelength of the photon.
times longer than the wavelength of the photon.
E) the wavelength of the electron will be about 1800 times longer than the wavelength of the photon.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The electron configuration of a ground-state copper
Q91: Breaking the oxygen-oxygen bond in hydrogen peroxide
Q96: If we take away two electrons from
Q97: What is the wavelength (nm)for a photon
Q98: Write the ground state electron configuration for
Q99: What is the energy in joules of
Q100: Using the figure below, categorize electromagnetic radiation
Q111: How many unpaired electrons does an atom
Q121: Which of the following atoms is diamagnetic
Q130: Each shell (principal energy level) of quantum