Multiple Choice
Which one of the following sets of quantum numbers is not possible? 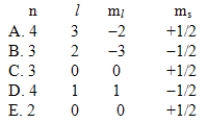
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: The colors of the visible spectrum are
Q59: When the electron in a hydrogen
Q60: What is the maximum number of electrons
Q61: The quantum numbers, n = 3, l
Q65: Calculate the wavelength of a neutron that
Q66: In an electron microscope, electrons are accelerated
Q67: Which ground-state atom has an electron configuration
Q68: In the following diagram of a wave
Q72: The frequency of the emitted light from
Q109: What is the wavelength of radiation that