Multiple Choice
Which of the elements listed below has the following pattern for its first six ionization energies? (I1 = first ionization energy, I2 = second ionization energy, etc.) 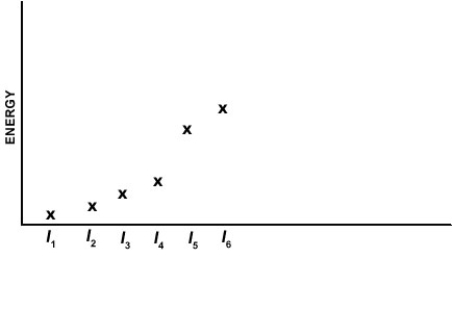
A) Ca
B) Si
C) Al
D) Se
E) P
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: Which pair of elements from different groups
Q55: How many electrons are in the 4p
Q57: As opposed to early periodic tables based
Q69: How many 3d electrons does an Fe<sup>3+</sup>
Q105: The electron affinity of fluorine is essentially
Q110: Write the ground-state electron configuration for Al<sup>3+</sup>.
Q113: Write the ground-state electron configuration for Cr<sup>3+</sup>.
Q115: What are the products of the
Q119: For which of the following reactions
Q133: How many core electrons does a chlorine