Solved
Calculate the Energy Change for the Reaction K(g)+ I(g) K+(g)+ I - (G)
Given the Following Ionization Energy (IE)and
Multiple Choice
Calculate the energy change for the reaction K(g) + I(g) K+(g) + I - (g)
Given the following ionization energy (IE) and electron affinity (EA) values. 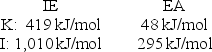
A) -124 kJ/mol
B) -715 kJ/mol
C) 715 kJ/mol
D) 1429 kJ/mol
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Which of the following ionic solids would
Q32: Use the Born-Haber cycle to calculate
Q33: What is the formal charge on phosphorus
Q36: Use the bond enthalpy data given to
Q42: What type of bonding is present in
Q45: Which of the following ionic solids would
Q47: Which of the elements listed below is
Q67: The Lewis structure reveals a double bond
Q68: How many covalent bonds will be drawn
Q97: Assuming the octet rule is obeyed, how