Multiple Choice
Use the Born-Haber cycle to calculate the standard enthalpy of formation ( H 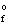 ) for LiCl(s) given the following data:
) for LiCl(s) given the following data:
H(sublimation) Li = 155.2 kJ/mol
I1 (Li) = 520 kJ/mol
Bond energy (Cl-Cl) = 242.7 kJ/mol
EA (Cl) = 349 kJ/mol
Lattice energy (LiCl(s) ) = 828 kJ/mol
A) 440 kJ/mol
B) 320 kJ/mol
C) -260 kJ/mol
D) -380 kJ/mol
E) -1420 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Classify the Ca - Cl bond in
Q35: Which one of the following molecules has
Q52: The total number of valence electrons in
Q55: The bond in F<sub>2</sub> is described as
Q80: Which one of the following ionic solids
Q112: What is total number of lone pairs
Q114: Dichloromethane, CH<sub>2</sub>Cl<sub>2</sub>, is an important solvent in
Q115: Write a Lewis structure for the chlorate
Q119: Use the bond enthalpy data given to
Q120: Write the Lewis structure for the product