Multiple Choice
Arrange the following bonds in order of increasing ionic character 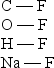
A) C - F < O - F < H - F < Na - F
B) C - F < H - F < O - F < Na - F
C) O - F < C - F < H - F < Na - F
D) H - F < C - F < O - F < Na - F
E) Na - F < H - F < C - F < O - F
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: The Si - Cl bond has less
Q15: Arrange the following bonds in order of
Q17: The Lewis dot symbol for the S<sup>
Q25: List all types of bonding present in
Q58: A molecule with two resonance structures is
Q88: Of the species NO<sub>2</sub>, NO, and N<sub>2</sub>,
Q93: Classify the C - Cl bond in
Q120: Of the following substances, KCl, KBr, and
Q122: Which of the following solids would have
Q133: How many covalent bonds will be drawn