Multiple Choice
Which of the following Lewis structures is incorrect?
A) 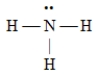
B) 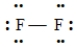
C) 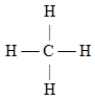
D) 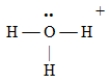
E) 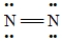
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The Lewis structure reveals an unpaired electron
Q32: A nonpolar covalent bond (i.e., pure covalent)
Q53: List all types of bonding present in
Q56: Write a Lewis structure for SO<sub>3</sub> that
Q58: What type of bonding is present in
Q59: Write a Lewis structure for the chlorite
Q101: The covalent bond with the greatest polarity
Q103: Arrange the following bonds in order of
Q117: Which of the elements listed below would
Q128: Nitrous oxide, N<sub>2</sub>O, is sometimes called "laughing