Multiple Choice
The number of pi bonds in the molecule below is 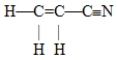
A) 1
B) 2
C) 3
D) 5
E) 9
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Give the number of lone pairs around
Q45: The hybridization of B in the BF<sub>3</sub>
Q92: The geometry of the hybrid orbitals about
Q95: The F - S - F bond
Q96: What is the hybridization of the As
Q96: The bond angles in SF<sub>5</sub><sup>+</sup> are expected
Q101: The bond angles in CO<sub>3</sub><sup>2-</sup> are expected
Q102: According to VSEPR theory, which of the
Q116: Give the number of lone pairs around
Q143: A molecule with 3 single bonds and