Multiple Choice
In the following picture, each arrow represents a molecule or atom.Based on the arrangement in the solid state as shown, which of the following best represents the unit cell? 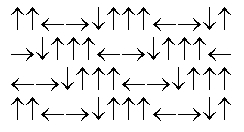
A) 
B) 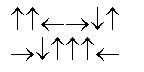
C) 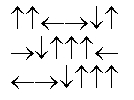
D) 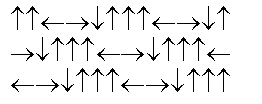
E) 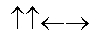
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q80: A phase change from the gas phase
Q85: Ethanol (C<sub>2</sub>H<sub>5</sub> - OH) will have a
Q125: The molar heats of sublimation and fusion
Q126: The meniscus for water is curved upward
Q127: What phase exists at the point labeled
Q128: Which of the following liquids would have
Q131: Which would be expected to have the
Q132: Which would have the higher boiling point
Q133: The normal boiling point of methanol (CH<sub>3</sub>OH)is
Q140: Platinum has a face-centered cubic crystal structure