Multiple Choice
Use the graph of vapor pressure to determine the normal boiling point of O2. 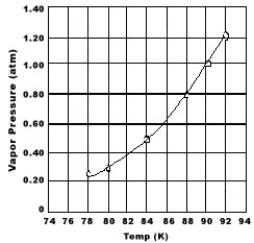
A) 84 K
B) 88 K
C) 90 K
D) 92 K
E) O2 doesn't boil because it is always a gas.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: Which would have the stronger intermolecular forces
Q36: Find the temperature at which ethanol boils
Q38: Polyethylene plastic consists of long chains of
Q40: Use the following data to determine the
Q42: Identify the dominant (strongest)type of intermolecular force
Q44: The specific heat of liquid ethanol, C<sub>2</sub>H<sub>5</sub>OH(l),
Q57: Arrange the following substances in order of
Q91: The shape of the water-to-glass meniscus results
Q120: All intermolecular forces must be overcome in
Q134: Which one of the following substances should