Multiple Choice
How much energy (heat) is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? 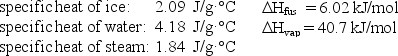
A) 40.2 kJ
B) 157.8 kJ
C) 1,086 kJ
D) 2,570 kJ
E) 22,957 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Which one of the following substances should
Q32: Which of the following is not true
Q50: Identify the dominant (strongest)type of intermolecular force
Q51: An example of a covalent network solid
Q53: Calculate the amount of heat that
Q56: Indicate all the types of intermolecular forces
Q59: Given the following liquids and their boiling
Q60: Which of the following constants is/are
Q74: Which one of the following substances crystallizes
Q148: Which of the following phase changes is