Multiple Choice
The isomerization of cyclopropane to form propene is a first-order reaction. 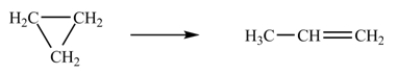 At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min.What is the half-life of cyclopropane at 760 K?
At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min.What is the half-life of cyclopropane at 760 K?
A) 3.4 × 10-2 min
B) 2.5 min
C) 23 min
D) 29 min
E) 230 min
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: B is a catalyst in the following
Q42: It takes 42.0 min for the concentration
Q47: The following initial rate data apply
Q48: Given that E<sub>a</sub> for a certain biological
Q49: Nitric oxide reacts with chlorine to
Q50: For the chemical reaction system described by
Q51: Concerning the decomposition of A, A
Q105: The half life for a first order
Q113: The reaction A + 2B
Q124: A reaction is experimentally found to follow