Multiple Choice
At 700 K, the rate constant for the following reaction is 6.2 × 10-4 min-1. 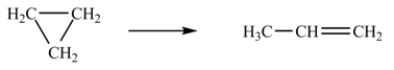 How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene?
How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene?
A) 1,120 min
B) 360 min
C) 3710 min
D) 1.4 × 10-4 min
E) 280 min
Correct Answer:

Verified
Correct Answer:
Verified
Q31: The thermal decomposition of acetaldehyde, CH<sub>3</sub>CHO
Q33: The rate determining step must be the
Q36: At a certain temperature, the data
Q37: For the overall chemical reaction shown below,
Q38: Nitrous oxide (N<sub>2</sub>O)decomposes at 600°C according
Q40: For the reaction BrO<sub>3</sub><sup>- </sup>+ 5Br<sup>-</sup>+
Q63: In general, to calculate the activation energy
Q83: A reaction was experimentally determined to follow
Q90: The reaction C<sub>4</sub>H<sub>10</sub> <span class="ql-formula"
Q97: The first-order decomposition, A <span