Multiple Choice
The graphs below all refer to the same reaction.What is the order of this reaction? 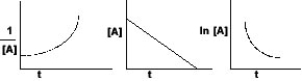
A) zero order
B) first order
C) second order
D) unable to predict
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q82: Concerning the rate law, Rate = k[A][B],
Q120: The rate law predicted by the following
Q121: With respect to the figure below, which
Q122: When the concentrations of reactant molecules are
Q122: Chlorine dioxide reacts in basic water
Q124: The isomerization of cyclopropane follows first order
Q125: Aspirin, C<sub>9</sub>H<sub>8</sub>O<sub>4</sub>, slowly decomposes at room
Q126: For the reaction X + Y
Q128: A certain first-order reaction A
Q130: Nitric acid is formed by the