True/False
The rate determining step in the following mechanism is bimolecular 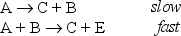
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: At a particular temperature the first-order
Q11: In general, to calculate the time required
Q38: Complete the following statement: A catalyst<br>A) increases
Q48: Concerning the rate law, Rate = k[A]<sup>2</sup>[B],
Q57: For the reaction whose rate law is
Q77: The following mechanism has been suggested
Q79: The rate law for the reaction
Q83: For the first-order reaction 2N<sub>2</sub>O<sub>5</sub>
Q85: Given the rate law for a reaction,
Q120: For the chemical reaction A