Multiple Choice
In the reaction: CH3COOH(aq) + NH2- (aq) 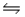 CH3COO- (aq) + NH3(aq) , the conjugate acid-base pairs are:
CH3COO- (aq) + NH3(aq) , the conjugate acid-base pairs are:
A) pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3
B) pair 1: CH3COOH and NH3; pair 2: NH2- and CH3COO-
C) pair 1: CH3COOH and NH2- ; pair 2: NH3 and CH3OO-
D) pair 1: CH3COOH and CH3COO- ; pair 2: NH4+ and NH3
E) pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3+
Correct Answer:

Verified
Correct Answer:
Verified
Q27: If the pH of tomato juice is
Q29: In aqueous solutions at 25°C, the sum
Q30: What mass of potassium hypochlorite must be
Q31: Predict the direction in which the equilibrium
Q34: What mass of sodium formate (HCOONa)must be
Q35: Aspartic acid (C<sub>4</sub>H<sub>7</sub>NO<sub>4</sub>), one of the 20
Q36: Which of these acids is stronger, H<sub>3</sub>AsO<sub>3</sub>
Q37: A 0.10 M HF solution is 8.4%
Q39: Identify the conjugate acid of HCO<sub>3</sub><sup>-</sup>.<br>A) H<sub>2</sub>O<br>B)
Q119: What is the pH of a 0.080