Multiple Choice
In the reaction: 2H2O(l) 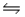 H3O+(aq) + OH- (aq) the conjugate acid-base pairs are
H3O+(aq) + OH- (aq) the conjugate acid-base pairs are
A) pair 1: H2O and H2O; pair 2: H3O+ and OH-
B) pair 1: H3O+ and OH-; pair 2: H3O+ and H2O
C) pair 1: H3O+ and OH-; pair 2: OH- and H2O
D) pair 1: H2O and OH-; pair 2: H2O and H3O+
E) pair 1: H3O+ and HO-: pair 2: OH- and H3O+
Correct Answer:

Verified
Correct Answer:
Verified
Q79: Arrange the acids HOCl, HClO<sub>3</sub>, and HClO<sub>2</sub>
Q106: Which one of these salts will form
Q112: Which of the following does not fit
Q117: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img
Q118: Calculate the H<sup>+</sup> ion concentration in a
Q121: The OH<sup>-</sup> concentration in a 1.0 ×
Q122: Write the formula for the conjugate base
Q124: The pH of a Ba(OH)<sub>2</sub> solution is
Q125: Arrange the acids HBr, H<sub>2</sub>Se, and H<sub>3</sub>As
Q149: Which solution will have the lowest pH<br>A)