Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction H2CO3 + F-
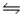
HCO3- + HF.Ka1( H2CO3) = 4.2 × 10-7; Ka(HF) = 7.1 × 10-4
A) to the right
B) to the left
C) in the middle
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Which one of the following salts will
Q51: The hydrolysis of NH<sub>4</sub>F will result in
Q54: Which of the following is both a
Q67: A 0.14 M HNO<sub>2</sub> solution is 5.7%
Q68: The pH of a sample of river
Q71: Arrange the acids H<sub>2</sub>Se, H<sub>2</sub>Te, and H<sub>2</sub>S
Q73: HCN is classified as a weak acid
Q74: Calculate the concentration of chromate ion (CrO<sub>4</sub><sup>2-</sup>)in
Q76: The OH<sup>-</sup> concentration in a 2.5 ×
Q143: Which solution will have the lowest pH<br>A)